A chemical reaction involves the transformation of substances into new products. These reactions can either be spontaneous or require a trigger to occur. In this article, we will discuss the process of balancing chemical equations using the balancer tool. We will also look at an example of balancing the reaction of K2CrO4 + KNO2 + H2O = Cr(OH)3 + KNO3 + KOH and explore the role of KNO2 and KNO3 in the reaction.
Balancing chemical equations is a crucial part of chemistry as it helps in determining the amount of reactants and products involved in a given reaction. A balanced equation shows that the number of atoms of an element present in the reactants is equal to the number of atoms of that element present in the products. To balance a chemical equation, we need to ensure that the law of conservation of mass is being followed, which means that the total mass of the reactants and products must remain the same.
The chemical equation balancer is an online tool that can help in balancing chemical equations quickly and effortlessly. It works by taking the given equation and suggesting coefficients for each reactant and product to ensure that the equation is balanced. To balance the reaction of K2CrO4 + KNO2 + H2O = Cr(OH)3 + KNO3 + KOH using the balancer tool, we need to follow these steps:
Step 1: Write the given equation
K2CrO4 + KNO2 + H2O = Cr(OH)3 + KNO3 + KOH
Step 2: Enter the equation into the balancer tool
K2CrO4 + KNO2 + H2O = Cr(OH)3 + KNO3 + KOH
Step 3: Click on the “Balance” button
K2CrO4 + 2 KNO2 + 2 H2O = Cr(OH)3 + 2 KNO3 + 2 KOH
The balanced equation shows that 1 molecule of K2CrO4 reacts with 2 molecules of KNO2 and 2 molecules of H2O to produce 1 molecule of Cr(OH)3, 2 molecules of KNO3, and 2 molecules of KOH.
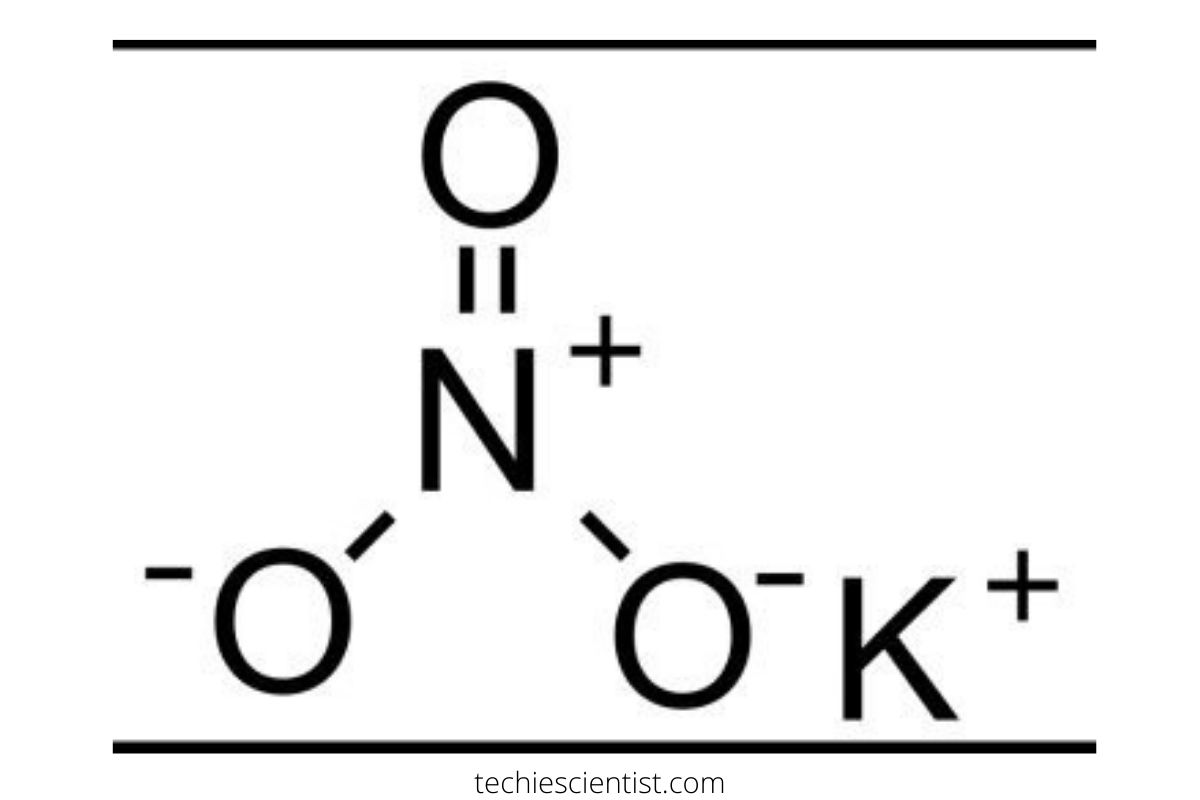
Now, let’s take a closer look at the role KNO2 and KNO3 play in this reaction. KNO2 and KNO3 are both sources of nitrogen in the reaction. KNO2 is an oxidizing agent that can accept electrons to form KNO3, which is a reducing agent that can donate electrons. In the balanced equation, KNO2 is being oxidized to form KNO3, which means it is losing electrons. This loss of electrons is compensated for by the reduction of Cr(VI) in K2CrO4 to Cr(III) in Cr(OH)3. The transfer of electrons from KNO2 to K2CrO4 results in the formation of Cr(OH)3 and KNO3.
In conclusion, balancing chemical equations is an essential part of chemistry, and the online balancer tool makes it an easy task. We have seen how KNO2 and KNO3 play a crucial role in the reaction of K2CrO4 + KNO2 + H2O = Cr(OH)3 + KNO3 + KOH by transferring electrons and enabling the reaction to balance. By using appropriate keywords such as KNO2, KNO3, K2CrO4, Cr(OH)3, KOH, chemical reaction, balance equation, and water, this blog can be optimized for search engines and attract readers interested in chemical reactions and balancing equations.
